 |
| Dear Peers, |
| With the vigorous development of biotechnology, innovative biopharma and biosimilars in Asia have set sail. However, 2019 opportunities and challenges coexist: |
Under the new medical reform program, who will take the lead in listing?
In the face of severe international competition, how can enterprises differentiate the distribution of the biopharmaceutical market?
How to implement clinical R&D and compliance management to speed up registration and seize the market?
Which cutting-edge approach, technology application and process optimization can help enterprises form robust processes, improve productivity and ensure quality?
How to control the life-cycle of the products to avoid risks, reduce costs and accelerate commercial production? |
| " 6th BioCon China 2019 " closely follow the latest development and challenges in the industry. It will invite 70 + global experts and scholars, enterprise leaders and leading technical elites to focus on the latest regulatory and policy developments of biopharmaceuticals, protein engineering design and structural optimization, clinical development and design strategies, challenges in quality control and characterization verification, commercial productivity improvement and market layout, application and prospect of cutting-edge technologies, so as to promote industry-university-research collaboration and accelerate Asian Biopharmaceutical listing process. |
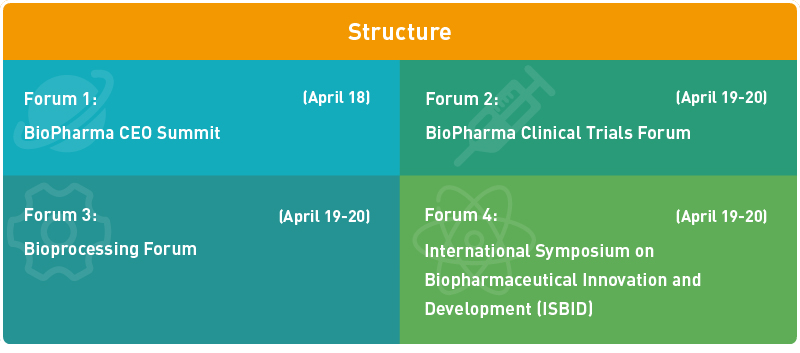 |
| News in BioPharma CEO Summit Highlights |
- Tracking the latest developments in biopharmaceutical policy guidance and review regulations in Asia
- The breakdown of biosimilars and innovative biopharma in international competition under ICH
- Looking ahead to the future strategy for NDA approval and market access of Asian pharmaceutical enterprises
- Discussing the patent protection and evasion strategy of product life cycle
|
| Highlights in BioPharma Clinical Trials Forum |
- Grasping the leading practice and experience of phase III clinical development of biopharmaceuticals in the European and American standardized market
- Learning the clinical similarity research and compliance management of biosimilars in leading pharmaceutical enterprises
- Exploring the differentiation strategy and experimental design of clinical indication development of innovative biopharma ( PD - 1 / L1, etc. )
- Summarizing the clinical management and quality control experience of macromolecular drugs to improve the clinical operation efficiency
|
Hotshots in Bioprocessing Forum |
- Mastering the quality and compliance requirements of biopharmaceuticals under international cGMP regulations
- Listening to the summary of successful upstream large-scale cell culture process amplification to improve yield and quality robustness
- Seizing the identification of complex impurities in downstream processes and leading separation and purification methods
- Learning the leading practices of upstream and downstream process characterization, continuous verification and tech transfer in commercialization
|
Focus of The 5th International Symposium on Biopharmaceutical Innovation and Development |
- Following up the latest discovery of immuno-oncology and hot targets in antibody study and early research of new drugs
- Learning the leading practice and technology platform for the design and engineering optimization of bispecific antibodies
- Appreciating the frontier application of structural analysis and detection technology of macromolecular complex proteins
- Listening to the latest technology and leading practices of novel formulations for biotherapeutic protein drugs
|
| Previous Distinguished Speakers |
 |
|
 |
|
 |
|
 |
Steve Drew
Member of Academic of Engineering USA, Former VP, Merck |
|
Rolf G. Werner
Corporate Senior Vice President bei Boehringer Ingelheim |
|
John Gilly
GMember of Academic of Engineering USA, Former VP, Merck |
|
Niklas Ekman
Senior Researcher and Quality (CMC) Assessor, Finnish Medicine Agency, Finland Biosimilar Medicinal Products Working Party (BMWP), EMA |
 |
|
 |
|
 |
|
 |
Angela Thomas
Vice-chair of the Commission for Human Medicines (CHM), MHRA |
|
Jonathan Sprent
Professor, Garvan Institute of Medical Research Sydney, Australia |
|
Venke Skibeli
Senior Scientist and Clinical Assessor, Norwegian Medicines Agency |
|
Elena Wolff-Holz
Chairman of the Biosimilar Medicines Working Party, EMA |
 |
|
 |
|
 |
|
 |
Patrick Liu
Vice President of Global Biologics R&D and Head of Biologics, Assays and Technology, Teva Pharmaceuticals U.S.A |
|
Hans-Martin Mueller
Director, Bioprocess Development, Merck |
|
Katrin Rupalla
Head of R&D in China, BMS |
|
Youchun Wang
Deputy Director, National Institutes for food and drug Control |
|
| Distinguished Speakers |
• Alexey Rak, Head of Bio Structure and Biophysics, Sanofi
• Jiu-Li Song, Principle Scientist, Amgen
• Donald Palahnuk, Vice President, Bio-Thera Solutions Ltd
• Marcus Fiadeiro, Associate Director, DSP Continuous Manufacturing, Sanofi
• Martin Schiestl, Chief Science Officer bei Sandoz
• David Shen, SVP and Head of Biologics Research and CMC, NGM Biopharmaceuticals
• Jianwei Zhu, Dean, School of Pharmacy, SJTU
• Scott Liu, Co-founder, President and CEO at Shanghai Henlius Biotech Inc.
• Joe Zhou, CEO of Genor Biopharma Co. Ltd.
• Daotian Fu, Vice President & Managing Director of Livzon Pharmaceutical Group
• Rong Chen, Chief Medical Officer of JHL Biotech
• Xiangyang Zhu, CEO, Huabo Bio
• Junli Zhang, COOЃЌShanghai Henlius Biotech Inc.
• Joan Huaqiong Shen, President of R&D, I-Mab
• Wei Xu, SVP at Innovent Biologics, Inc.
• Jian Ni, CEO, Nuance Biotech (Shenzhen) Co. Ltd., President of R & D and CSO , Nuance Biotech Inc.
• Claudia Lin, CEO, JADE BioMedical SVP, Head of CMC, Harbour BioMed
• Hui Feng, COO, Junshi Bio
• Jason Li, Vice President, Head of CMC development at EpimAb Biotherapeutics, Inc.
• Jerry Su, Zhejiang Huahai Biopharmaceuticals Co., Ltd, A Subsidary of Huahai Pharm
• Xiaoyu Yang, Principal Scientist, Merck
• Chen Zhiqiang, Director of Process and Analytical Development, and Head of CMC at Adagene Inc.
• Jun Johnny Yang, Associate Director, Ribon Therapeutics Director, Shanghai Chest Hospital
• Zhaoxiang Ran, VP, Teruisipharm
• Jing Li, VP, Mabpharm
• Shengfeng Li, ExecutiveЃЌBio-Thera Solutions
• Jingrong Li, SVPЃЌCStone Pharmaceuticals
• Hongtao Lu, Co-Founder and CSO at Elpiscience Biopharma
• Hi Yan, Co-founder, REMD Biotherapeutics Inc
...... |
| Testimonials |
• Very satisfied with the event and full of harvest --- Sanofi
• Speakers are very professional, and the conference is well organized with focusing on detail. --- Boehringer Ingelheim Biopharmaceutical (China) Co., Ltd.
• The conference scale is relatively good, and it fits with our company very well. --- Sino Biological Inc.
• Very good peer exchange platform, which will accelerate the development of the domestic biomedical industry. ---GE
• Excellent event and management. The selection and content of the topic have been carefully considered and fit the popular issues in the industry. ---Zhejiang Teruisi Pharmaceutical Co., Ltd.
• Very good. Speakers and attendees are all from well-known organization in the industry. Hence it is very helpful in opening practical ideas up. ---XINTRUM Pharmaceuticals, Deputy Director of Technology
|
| BioCon, as the annual event of the biopharmaceutical industry, focuses on industry hotspots and in-depth discussions after three months intensively investigation every. At present, it has accumulated more than 2,400 industry participants, over 260 industry speakers and in excess of 180 industry sponsors. |
| The 6th BioCon China 2019 is also in full swing. If you want to know more about the forum (become speaker, become sponsor, and become Delegate), please call the organizing committee immediately: +86 18017939885 or email us at biocon@bmapglobal.com. |
Contact us
T: +86 180 1793 9885
E: biocon@bmapglobal.com
W: www.bmapglobal.com/biocon2019 |
|
 > БГРА Йз ЧрЛч > ТќАЁНХУЛ
> БГРА Йз ЧрЛч > ТќАЁНХУЛ
 > БГРА Йз ЧрЛч > ТќАЁНХУЛ
> БГРА Йз ЧрЛч > ТќАЁНХУЛ