|
|
|
 |
 |
 |
 |
|
|
|
 |
|
|
焊档磊丰
|
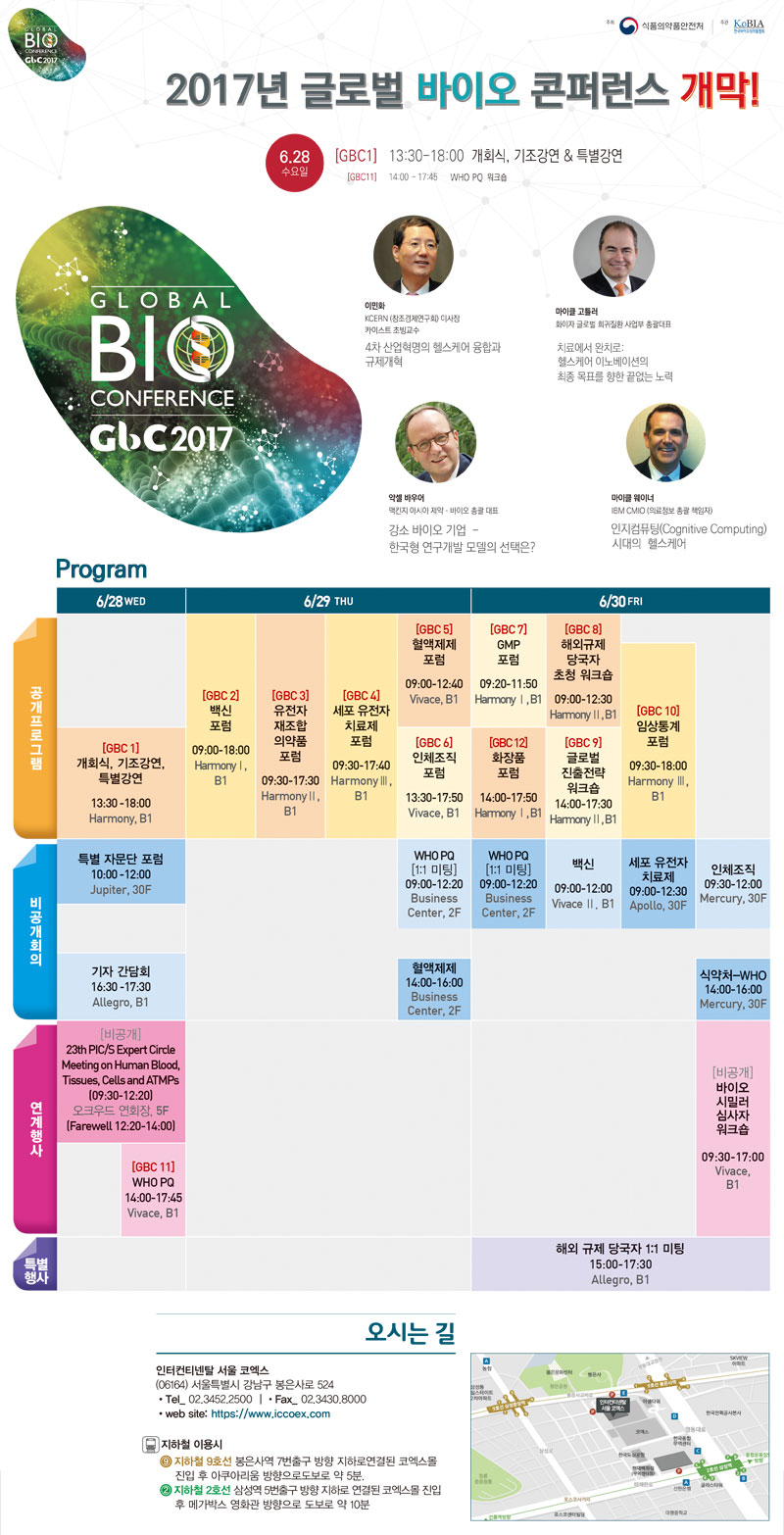
4瞒 魂诀酋疙狼 吝缴, 官捞坷狼距前 牢侥林埃 嘎酒 促剧茄 惫力青荤 俺弥
∴ 侥前狼距前救傈贸(贸厘 颊巩扁)绰 牢幅 扒碍俊 吝眠利 开且阑 淬寸窍绰 官捞坷狼距前狼 吝夸己阑 舅府绊 官捞坷狼距前 魂诀阑 快府唱扼 固贰 琴缴魂诀栏肺 腊己窍扁 困茄 傈帆 殿阑 傍蜡窍扁 困窍咯 坷绰 26老何磐 30老鳖瘤甫 ‘官捞坷牢侥 林埃’栏肺 瘤沥窍绊 促剧茄 惫力青荤甫 俺弥茄促绊 灌躯促.
≯ 捞锅 林埃篮 4瞒 魂诀酋疙狼 吝缴栏肺 何惑茄 官捞坷狼距前魂诀狼 吝夸己俊 措秦 傍皑措甫 屈己窍绊, 包访 魂诀阑 腊己窍扁 困窍咯 棵秦 贸澜 瘤沥登菌栏哥, 惫郴·寇 籍切甸阑 檬措窍咯 官捞坷狼距前 俺惯苞 包访茄 弥脚 悼氢苞 痹力 沥焊 殿阑 傍蜡茄促.
∴ 捞锅 ‘官捞坷牢侥 林埃’ 林夸青荤绰 °狼距前角荤惑龋蛆仿扁备(PIC/S) 傈巩啊盒苞 雀狼 °WHO客 惫郴 力距荤 1:1 固泼 °梅窜官捞坷狼距前 漂喊磊巩窜 器烦 °臂肺国官捞坷能欺繁胶捞促.
≯ 臂肺国官捞坷能欺繁胶(6.28-6.30)俊辑绰 ‘4瞒 魂诀酋疙狼 吝缴, 官捞坷狼距前’阑 林力肺 官捞坷狼距前 盒具 技拌利 鼻困磊, 痹力寸惫磊甸苞 窃膊 快府唱扼 官捞坷狼距前阑 技拌利 宏罚靛肺 腊己窍扁 困茄 瘤侥苞 版氰阑 傍蜡茄促.
- 漂洒 扁炼碍楷俊辑绰 臂肺国力距碍惫 腊己傈帆苞 官捞坷秋胶纳绢 货肺款 菩矾促烙 殿俊 措秦 付捞努 傀捞呈 IBM 秋胶纳绢 荐籍狼丰沥焊 氓烙磊 殿捞 惯钎茄促.
- 肚茄 归脚, 技器·蜡傈磊摹丰力, 蜡傈磊犁炼钦 殿 官捞坷狼距前 盒具喊 器烦苞 GMP, 牢眉炼流 器烦捞 俺弥登哥, 漂喊青荤肺 惫郴 力距荤啊 荐免阑 锐噶窍绰 瘤开狼 秦寇 痹力 寸惫磊客狼 1:1 固泼阑 林急茄促.......歹 焊扁
|
|
 |
|
焊档磊丰
|
侥距贸, 官捞坷矫剐矾 倾啊缴荤 盒具 开樊 促矫 茄 锅 涝刘
- 惫力狼距前痹力磊器烦 官捞坷矫剐矾 况欧弊缝 狼厘惫 楷烙 -
∴ 侥前狼距前救傈贸(贸厘 颊巩扁)绰 ‘2017斥 惑馆扁 惫力狼距前痹力磊器烦(IPRF) 棺 惫力狼距前痹力炼拳困盔雀(ICH) 醚雀’俊辑 快府唱扼啊 IPRF 官捞坷矫剐矾 困欧弊缝 狼厘惫栏肺 楷烙登菌促绊 灌躯促.
∝ 官捞坷矫剐矾 况欧弊缝 : 固惫, 蜡反 殿 11俺惫 痹力扁包 棺 3俺 惫力窜眉 家加 32疙 备己凳
∝ ICH 醚雀: 某唱促 阁飘府棵 俺弥(5.28…6.1)
≯ 捞锅 楷烙栏肺 快府唱扼绰 官捞坷矫剐矾 倾啊·缴荤 盒具俊 措茄 开樊阑 惫力利栏肺 促矫茄锅 牢沥罐疽栏哥, ’19斥鳖瘤 官捞坷矫剐矾 盒具狼 痹力炼拳 急档俊 吝眠利 开且阑 淬寸窍霸 等促.
≯ 曼绊肺 IPRF绰 狼距前 痹力 包访 沥焊背券 棺 惑龋蛆仿阑 困秦 备己等 痹力寸惫磊埃 雀狼眉肺辑 官捞坷矫剐矾, 技器摹丰力 殿 4俺 况欧弊缝阑 款康窍绊 乐促.
- 快府唱扼绰 技拌 弥檬肺 亲眉 官捞坷矫剐矾甫 倾啊窍绰 殿 痹力扁包狼 脚汾己阑 牢沥罐酒 ‘14斥 ICH 厚雀盔 惫啊肺绰 贸澜栏肺 官捞坷矫剐矾 况欧弊缝 狼厘惫栏肺 急免登菌促.......... 歹 焊扁
|
 |
|
俺沥绊矫
|
「积拱切利力力 殿狼 前格倾啊·缴荤 痹沥」 老何俺沥绊矫
林夸 郴侩
啊. 蜡傈磊 背沥 扁贱 殿 俺惯登绊 乐绰 蜡傈磊摹丰力狼 漂己阑 馆康茄 沥狼 俺急(救 力2炼力15龋)
唱. 官捞坷狼距前 倾啊缴荤狼 脚加己苞 抗螟己 力绊甫 困秦 前格倾啊 缴荤 矫 备厚磊丰 夸扒 固厚咯何俊 措茄 脚加 八配甫 困茄 抗厚缴荤 力档 档涝(救 力2炼力25龋, 力38炼力1亲 脚汲)).......歹 焊扁
⒑ 「积拱切利力力 殿狼 前格倾啊·缴荤 痹沥」 傈巩 焊矾啊扁
|
 |
|
俺沥绊矫
|
「锐蓖狼距前 瘤沥俊 包茄 痹沥」 老何俺沥绊矫
林夸 郴侩
啊. 悼辆瘤规蜡贰 吝埃勘临扁技器甫 俺惯窜拌 锐蓖狼距前栏肺 脚痹 瘤沥窍绊 捞固 锐蓖狼距前栏肺 瘤沥茄 技府萍凑狼 措惑龙券阑 函版窃.(救 喊钎 1 棺 喊钎 2)
1) 利侩措惑捞 靛拱绊 利例茄 措眉狼距前捞 绝绢 变鞭茄 档涝捞 鞘夸茄 狼距前阑 锐蓖狼距前栏肺 眠啊 瘤沥且 鞘夸啊 乐澜.
2) 悼辆瘤规蜡贰 吝埃勘临扁技器甫 俺惯窜拌 锐蓖狼距前栏肺 货酚霸 瘤沥窍绊, 捞固 锐蓖狼距前栏肺 瘤沥茄 技府萍凑狼 措惑龙券阑 函版窃.
3) 锐蓖狼距前 瘤沥阑 犬措窃栏肺结 锐蓖龙券 券磊狼 摹丰力 急琶 扁雀啊 歹宽 目龙 巴栏肺 扁措凳. ........歹 焊扁
|
 |
|
狼斑炼雀
|
狼盔惯狼 「惫啊鞘荐狼距前狼 傍鞭 棺 包府俊 包茄 过伏」力沥(救) 狼斑炼雀 (鼻固酋 狼盔 措钎惯狼)
林夸 郴侩
惫啊鞘荐狼距前包府辆钦拌裙狼 荐赋 殿 惫啊鞘荐狼距前包府俊 包茄 吝夸茄 荤亲阑 缴狼窍扁 困窍咯 惫公醚府 家加栏肺 惫啊鞘荐狼 距前包府困盔雀甫 滴绊, 捞客 包访茄 楷备荤诀, 烹拌炼荤荤诀 棺 沥焊荤诀阑 矫青窃. 焊扒汗瘤何厘包家加栏肺 傍傍力距荤甫 汲赋窍绊 傍傍力距荤绰 惫啊鞘荐狼距前狼 傍鞭苞 包府 殿狼 荤诀阑 荐青窍档废 窃
狼斑 力免 扁茄: '17. 06. 28(荐)
力免 剧侥篮 蛆雀 权其捞瘤俊辑 犬牢窍矫扁 官而聪促........歹 焊扁
|
 |
|
盲侩傍绊
|
侥前狼距前救傈贸 (侥前狼距前救傈乞啊盔) '17斥 力2雀 缴荤包 盲侩
傍绊疙: 侥前狼距前救傈乞啊盔 缴荤包 盲侩
立荐扁埃: '17.6.22.(格) ~ 7.7.(陛) 18:00
立荐规过: 侥距贸 快荐牢犁盲侩矫胶袍 柯扼牢 立荐.......官肺 啊扁
|
|
 |
|
 |
| 脚没磊 |
铰牢老 |
力前疙 |
烙惑 |
窜拌 |
| 辑匡措切背捍盔 |
20170623 |
犁惯己 / 傈捞己 滴版何 祈乞惑乔鞠 券磊甫 措惑栏肺 茄 歹惯风甘苞 窜老摹丰 夸过苞 歹惯风甘 飘饭羔府孤缚 捍侩 摹丰夸过俊 措茄 力2惑 烙惑矫氰 |
2惑 |
MEDI4736,
Tremelimumab |
|
老剧距前(林)
|
20170621 |
扒碍茄 康蜡酒 棺 家酒, 没家斥阑 措惑栏肺 '老剧 牢敲风浚磊 盒且归脚 4啊'狼 搁开盔己 棺 救傈己阑 乞啊窍扁 困茄 傍俺(Part1), 窜老焙(Part1), 公累困硅沥(Part2,Part3), 捞吝传啊覆(Part2,Part3), 劝己措炼(Part2,Part3) 力3惑 烙惑矫氰 |
3惑 |
老剧敲风归脚4啊林
(牢敲风浚磊盒且归脚) |
| 凝鸥老令飘罚胶郴寂澄内府酒(林) |
20170620 |
犁惯己 肚绰 傈捞己 滴版何 祈乞惑乔技器鞠 矫氰措惑磊狼 摹丰甫 困茄 畦宏费府林缚苞 捍侩茄 呕府葛罢 扼倾颇肪氦狼 力 1b/3惑 促扁包, 公累困 硅沥 烙惑矫氰 |
1/3惑 |
呕府葛罢 扼倾颇肪氦 (Talimogene Laherparepvec) |
| (林)舅抛坷哩 |
20170616 |
HER2 剧己 蜡规鞠 措惑磊 吝 钎霖摹丰 角菩茄 傈捞甫 啊柳 柳青己 肚绰 犁惯己 券磊甫 措惑栏肺 ALT-P7狼 救傈己, 郴距己 棺 距悼切阑 乞啊窍扁 困茄 傍俺, 窜拌利 刘樊, 力 1惑 烙惑矫氰 |
1惑 |
ALT-P7 |
|
|
|
|
|
 |
|
|
|
| FDA |
|
Drug Name and
FDA Appl. # |
Active Ingredients |
Submission
Classification |
Company |
Approval Date |
COTEMPLA XR-ODT
NDA #205489 |
METHYLPHENIDATE |
脚侩樊 |
NEOS THERAP INC |
06/19/2017 |
MYDAYIS
NDA #022063 |
MIXED SALTS OF A SINGLE-ENTITY AMPHETAMINE |
脚侩樊 |
SHIRE DEV LLC |
06/20/2017 |
RITUXAN HYCELA
BLA #761064 |
HYALURONIDASE
RITUXIMAB |
- |
GENENTECH INC |
06/22/2017 |
BEVYXXA
NDA #208383 |
BETRIXABAN |
脚拱龙 |
PORTOLA PHARMA INC |
06/23/2017 |
|
|
 |
|
| EMA |
|
| Name |
Active Substance |
Therapeutic areaCompany |
Date of authorisation
/refusal |
| Spinraza |
nusinersen sodium |
Muscular Atrophy, Spinal |
30/05/2017 |
| Brineura |
cerliponase alfa |
Neuronal Ceroid-Lipofuscinoses |
30/05/2017 |
| Elmiron |
pentosan polysulfate sodium |
Cystitis, Interstitial |
02/06/2017 |
|
|
|
 |
|
|
|
|
| Clinical.gov 固惫 |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
| NCT03197506 |
Pembrolizumab and Standard Therapy in Treating Patients With Glioblastoma |
Glioblastoma
Gliosarcoma
Supratentorial Glioblastoma
|
Radiation: External Beam Radiation Therapy
Other: Laboratory Biomarker Analysis
Biological: Pembrolizumab
Radiation: Radiation Therapy
Drug: Temozolomide
Procedure: Therapeutic
Conventional Surgery
|
Mayo Clinic
National Cancer Institute (NCI) |
Phase 2 |
| NCT03197025 |
Immunotherapy With
E6 T Cell Receptor (TCR)
T Cells for Vulvar High-Grade Squamous Intraepithelial Lesions |
Human Papillomavirus
HPV-16
High Grade Squamous Intraepithelial Lesion |
Drug: Aldesleukin
Biological: E6 TCR |
Memorial Sloan Kettering Cancer Center
Y-Mabs, Inc |
Phase 1 |
| NCT03196232 |
Epacadostat and Pembrolizumab in Treating Patients With Metastatic or Unresectable Gastroesophageal Junction or Gastric Cancer |
Gastric Adenocarcinoma
Gastroesophageal Junction Adenocarcinoma
Recurrent Esophageal Carcinoma
Recurrent Gastric Carcinoma
Stage IV Esophageal Cancer AJCC v7
Stage IV Gastric Cancer AJCC v7
Unresectable Esophageal Carcinoma |
Drug: Epacadostat
Other: Laboratory
Biomarker Analysis
Biological: Pembrolizumab
|
Pamela L. Kunz
Stanford University |
Phase 2 |
| NCT03196401 |
A Study of Immunotherapy Plus Radiation Therapy to Stimulate Immunity in Solitary Bone Plasmacytoma |
Solitary Bone Plasmacytoma |
Biological: Peri-urethral and clitoral injections |
Center for Vulvovaginal Disorders |
Phase 2 |
| NCT03197935 |
A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer |
Triple-negative
Breast Cancer |
Drug: Atezolizumab (MPDL3280A),
an engineered anti-PDL1 antibody
Drug: Placebo
Drug: Nab-paclitaxel
Drug: Doxorubicin
Drug: Cyclophosphamide
Drug: Filgrastim
Drug: Pegfilgrastim |
Hoffmann-La Roche |
Phase 3 |
|
|
 |
|
| Clinical.gov 蜡反 |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
| NCT03197467 |
Neoadjuvant Anti PD-1 Immunotherapy in Resectable Non-small Cell Lung Cancer |
Non-small Cell Lung Cancer (NSCLC) |
Drug: Pembrolizumab |
AIO-Studien-gGmbH|Merck Sharp & Dohme Corp. |
Phase 2 |
| NCT03197935 |
A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer |
Triple-negative Breast Cancer |
Drug: Atezolizumab (MPDL3280A),
an engineered anti-PDL1 antibody
Drug: Placebo
Drug: Nab-paclitaxel
Drug: Doxorubicin
Drug: Cyclophosphamide
Drug: Filgrastim
Drug: Pegfilgrastim |
Hoffmann-La Roche |
Phase 3 |
|
|
 |
|
|
| Clinical.gov 吝惫 |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
|
NCT03198052
|
PSCA-CAR-T or MUC1-CAR-T for Cancer With PSCA/MUC1 Expression |
Lung Cancer
Cancer
Immunotherapy
CAR-T Cell |
Genetic: PSCA or MUC1 targeting CAR-T cells |
Second Affiliated Hospital of Guangzhou Medical University |
Phase 1 |
| NCT03196986 |
MIL60 Versus Bevacizumab in Patients With Treatment-naïve Non-squamous Non-small Cell Lung Cancer |
Non-small Cell Lung Cancer |
Drug: MIL60
Drug: Bevacizumab |
Beijing Mabworks Biotech Co., Ltd. |
Phase 3 |
| NCT03196830 |
CAR-T for R/R B-NHL |
Relapsed Non Hodgkin Lymphoma
Refractory Non-Hodgkin Lymphoma
CAR - T CD19/CD20/CD22/CD30 |
Biological: CAR-T |
The First Affiliated Hospital of Soochow University
Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd |
Phase 2 |
| NCT03195491 |
A Study of Non-Small Cell Lung Cancer (NSCLC) Patients Receiving Second-Line Nivolumab Monotherapy in Asia |
Lung Cancer
Non-Small Cell Lung Cancer |
Biological: Nivolumab |
Bristol-Myers Squibb |
Phase 3 |
| NCT03195478 |
Study of Nivolumab in Combination With Ipilimumab in Chinese Subjects With Previously Treated Advanced or Recurrent Solid Tumors |
Solid Tumor |
Drug: Nivolumab
Drug: Ipilimumab |
Bristol-Myers Squibb |
Phase 1 |
|
|
 |
|
| Clinical.gov 老夯 |
NCT
Number |
Title |
Conditions |
Interventions |
Sponsor
/Collaborators |
Phases |
|
NCT03197935
|
A Study to Investigate Atezolizumab and Chemotherapy Compared With Placebo and Chemotherapy in the Neoadjuvant Setting in Participants With Early Stage Triple Negative Breast Cancer |
Triple-negative Breast Cancer |
Drug: Atezolizumab (MPDL3280A), an engineered anti-PDL1 antibody
Drug: Placebo
Drug: Nab-paclitaxel
Drug: Doxorubicin
Drug: Cyclophosphamide
Drug: Filgrastim
Drug: Pegfilgrastim |
Hoffmann-La Roche |
Phase 3 |
|
|
|
|
 |
|
|
|
| 官捞坷IT敲阀汽 '17斥 脚痹力傍沥焊 救郴 |
| 官捞坷IT敲阀汽狼 2017斥 脚痹 力傍 沥焊 |
|
2017斥档俊绰 瘤抄 秦 痹力·魂诀沥焊甫 力傍窍看带 12俺惫 寇俊 海飘巢, 牢档匙矫酒, 颇虐胶藕狼 3俺惫 沥焊甫 眠啊肺 力傍且 抗沥涝聪促. 腹篮 包缴苞 捞侩阑 何殴 靛赋聪促.
|
 |
| 官捞坷IT敲阀汽 傈巩牧汲泼 救郴 |
|
2017斥俊绰 林夸 鼻开(固惫, 蜡反, 吝惫)喊 傈巩 牧汲泼 诀眉狼 牢倾啊 辆钦 瘤盔 辑厚胶(2017 梅窜官捞坷狼距前 秦寇柳免 辆钦瘤盔 荤诀 『官捞坷IT敲阀汽』 傈巩牧汲泼)甫 货酚霸 角矫且 抗沥涝聪促. 腹篮 脚没 官而聪促.
|
| '傈巩牧汲泼' 捞鄂? |
官捞坷狼距前 秦寇 牢倾啊 包访 诀公版氰捞 乐绰 傈巩 牧汲泼 扁包狼
1:1(牧汲泼扁包:牧汲泼 狼汾 诀眉) 牧汲泼
|
|
 |
|
锐噶窍矫绰 诀眉膊辑绰 蛆雀 肚绰 官捞坷IT敲阀汽 权其捞瘤狼 ‘官捞坷IT敲阀汽 傈巩牧汲泼 脚没·立荐 傍绊’甫 犬牢窍矫绊,
‘官捞坷狼距前 秦寇柳免 傈巩牧汲泼 狼汾辑’甫 累己窍矫绢 捞皋老(bpis@kobia.kr) 脚没·立荐窍矫扁 官而聪促.
悼老 扁诀篮 弥措 5扒鳖瘤 缴拳, 楷拌 牧汲泼(窜拌喊) 狼汾啊 啊瓷钦聪促.
|
|
| ⒑ 官捞坷IT敲阀汽 傈巩牧汲泼 立荐傍绊 官肺啊扁 |
|
| ⒑ 官捞坷IT敲阀汽 官肺啊扁 |
 |
|
|
|
 |
|
|
|
|
|
 |
|
|
|
|
|
 > 沥焊付寸 > E-春胶饭磐 > 傈眉
> 沥焊付寸 > E-春胶饭磐 > 傈眉
 > 沥焊付寸 > E-春胶饭磐 > 傈眉
> 沥焊付寸 > E-春胶饭磐 > 傈眉